Sibel Health, a leading digital health startup spun out of Northwestern University, has closed a $30 million Series C funding round, further solidifying its position in the patient monitoring space. Alongside this milestone, the company also celebrated its seventh FDA 510(k) clearance, signaling growing momentum for its advanced wearable technology.
The funding round was spearheaded by the Steele Foundation for Hope, with continued support from medical safety tech giant Dräger. Both have been long-standing backers of the Chicago-based health tech company.
Revolutionizing Patient Monitoring with ANNE One Platform
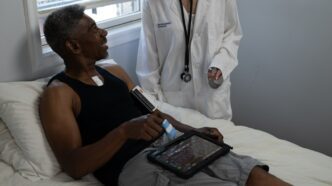
Sibel Health has developed the ANNE One platform, an FDA-cleared solution designed to transform patient monitoring. Equipped with clinical-grade wearable sensors, AI-powered analytics, and a cloud-based mobile platform, ANNE One provides continuous, wireless tracking of vital signs for patients aged 12 and older.
The platform features two distinct wearable components—Anne Limb and Anne Chest. The Anne Limb monitors skin and body temperature, while the Anne Chest captures heart rate, respiratory rate, skin temperature, step count, and fall detection. This dual-sensor system delivers comprehensive, real-time health data that empowers clinicians to detect early signs of deterioration.
With its latest FDA clearance, ANNE One now enables critical alarms and alerts through a centralized monitoring station—a significant upgrade aimed at improving patient safety in hospital settings.
Scaling Up: Sibel Health’s Growth and Expansion Plans
The new Series C funding will accelerate Sibel Health’s commercial rollout of the ANNE One system across the U.S. and Europe. According to co-founder and CEO Dr. Steve Xu, the company’s mission is to bring continuous monitoring to every hospital bed—a move he believes is long overdue.
“Most hospital beds in the U.S. lack continuous monitoring, even though over one million beds exist nationwide. What many don’t realize is that the majority of patient deaths happen outside the ICU, in unmonitored environments,” Dr. Xu explained. “This funding will help us expand rapidly while easing the workload for nurses.”
Sibel’s technology aims to fill a critical gap by offering constant, non-intrusive monitoring that could potentially reduce preventable deaths in general wards.
Strong Market Traction and Strategic Partnerships
Sibel Health has already begun making waves internationally. In December, the company inked a major partnership with the Capital Region of Denmark and Dräger to deploy its cutting-edge monitoring solutions in hospitals across Denmark.
This collaboration builds on Sibel’s earlier successes. Dräger led the company’s Series A round with a $10 million investment and remains a key strategic ally.
In 2023, Sibel Health launched Discovery, a specialized version of its monitoring platform tailored for clinical trials. The company also partnered with AI-driven clinical trial leader Medidata to enhance trial monitoring capabilities. That same year, Sibel secured FDA clearance for continuous vital sign monitoring of neonates and infants up to two years old—extending its reach to the most vulnerable patients.
Sibel’s journey has been marked by steady growth and investor confidence. Its previous Series B round raised $33 million in 2022, also led by the Steele Foundation for Hope.
With this fresh round of funding and another FDA nod, Sibel Health is now poised to transform how hospitals monitor patients globally. By bringing real-time, continuous vitals to every bed—not just the ICU—the company seeks to revolutionize healthcare delivery and minimize adverse patient outcomes.